What are valence electrons?
- See Full List On Sparknotes.com
- Solved: QUESTION 4 Valence Electrons Are Electrons Located ...
- Valence Electrons Are Electrons Located
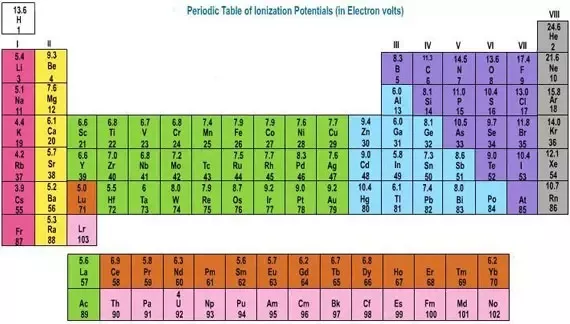
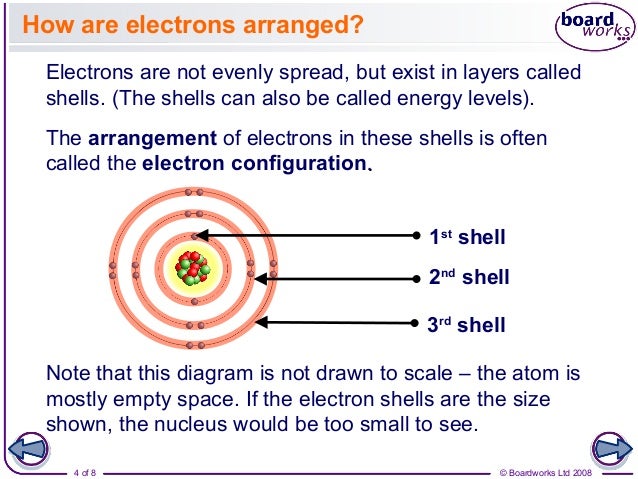
Valence electrons Electrons of an atom that have the highest energy (located in the last energy level). In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In non-metals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. Alence electrons are the electrons located in the outermost shell of an atom. For example, oxygen has six valence electrons and bromine has seven. Sodium has one valence electron and magnesium has two. You can get the number of valence electrons from the electron configuration. For example, carbon has this configuration: 1 s2 2 s2 2 p2.
5 Answers
The valence electrons are the electrons that determine the most typical bonding patterns for an element.
These electrons are found in the s and p orbitals of the highest energy level for the element.
Sodium
Sodium has 1 valence electron from the 3s orbital
Phosphorus
Phosphorus has 5 valence electrons 2 from the 3s and 3 from the 3p
Iron
Iron has 2 valence electrons from the 4s
Bromine
Bromine has 7 valence electrons 2 from the 4s and 5 from the 4p
Also, valence electrons is the electrons in the out most shell of an atom.
I hope this was helpful.
SMARTERTEACHER
Valence electrons are the outermost electrons and are therefore at the highest energy level.
Because they are the outermost energy levels, they are available to participate in chemical bonding, either ionic or covalent.
The alkali metals have one valence electron in their highest energy level.
The electron configuration for lithium is
Since the highest energy level for lithium is 2 and it contains one electron, the valence number for lithium is one.
Fluorine has a configuration of
The highest energy level for fluorine is 2 and this energy, it has 2 electrons in the s orbital and 5 electrons in the p orbital.
The total of valence electrons in the second energy level for this atom is 7 (2+ 5).

It is energetically favorable for lithium to lose one electron which is gained by fluorine.
As a consequence, lithium acquires a + 1 charge, while fluorine acquires a -1 charge.
These ions attract one another and form an ionic bond.
In summary, valence electrons determine the bonding patterns of atoms.
Here is a video which discusses how to draw Lewis structures for atoms showing their number of valence electrons.
Video from: Noel Pauller
Valence electrons are the electrons present in the outermost shell of an atom.
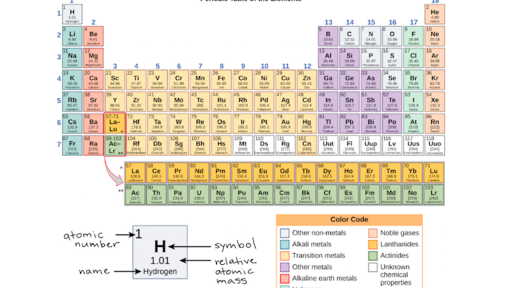
You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table.
For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively.
Atoms in Groups 13 and 18 have 3 and 8 valence electrons, respectively.
Valence electrons are responsible for the reactivity of an element. They determine how 'willing' the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron.
For example, alkali metals, which all have a valency of 1, want to lose that one electron and are likely to form ionic bonds (such as in the case of NaCl, or table salt) with a Group 17 element, which has a valency of 7 and wants to gain that one electron from the alkali metal (Group 1 element) to form a stable valency of 8.
For more on valence electrons and how they're related to the periodic table, I strongly recommend this video:
Citations: Tyler Dewitt. (2012, December 18) Valence Electrons and the Periodic Table [Video File].
Valence electrons are the outermost electrons in any atom. These are the electrons that are available for bonding with other atoms.
The number of valence electrons for a main group (group A) element is the same as the number of electrons in the s and p orbitals in the highest occupied energy level. A short-cut to determining this is to look at the group number on your periodic table.
The Roman numeral at the top of the group will tell you the number of valence electrons. If your periodic table has Arabic numerals for the group numbers, then look at the ones digit of the group number. This will match the number of valence electrons.
Here's how to count the valence electrons in transition metals.
See Full List On Sparknotes.com
Explanation:
A valence electron is an electron that is outside a noble-gas core and can be used to form bonds to other atoms.
Thus, the
The energy of an
However, the farther right an element is in each transition metal series, the closer the
Thus, in the
The remaining six elements (

The situation is even more complicated for the
For example,
In principle, it has ten valence electrons.
However, it never uses more than four of them.
Solved: QUESTION 4 Valence Electrons Are Electrons Located ...
It forms compounds like
Valence Electrons Are Electrons Located
Thus,
However, compounds of
Related questions

Comments are closed.